C02
Metabolic control of innate and adaptive immune responses to cutaneous Staphylococcus aureus infection
C02 (Vaeth) will investigate how metabolic competition between immune cells of the host and S. aureus shapes local immune responses. A second question will be how the uptake of glucose, which acts as the central nutrient, by host cells influences the spreading of S. aureus to secondary infection sites. M. Vaeth hypothesises that the epigenetic reprogramming of T cells by an altered glycolytic metabolism is a decision point that determines local immunity and systemic protection from S. aureus infections. Importantly, his group has shown previously that antigenic stimulation of T cells induces the expression of the high-affinity glucose transporter GLUT3. GLUT3-dependent glucose metabolism of T-cells controls the expression of major communication signals (IL-17, GM-CSF), indicating that GLUT3 also acts as a potential decision point during S. aureus infection. Based on this work, they aim to define the role of GLUT3 and other glucose transporters in the innate and adaptive immune system and investigate how alterations in intermediary glucose metabolism prevent or permit S. aureus spreading to secondary infection sites.
Project plan
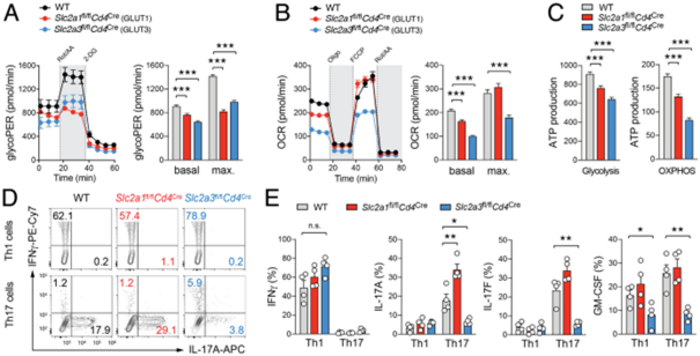
We hypothesise that aerobic glycolysis controls immunity to S. aureus and determines if the pathogen can spread from the primary infection niche to secondary sites. The premise that glucose uptake promotes innate and adaptive immune cell responses to S. aureus is supported by our preliminary data using mice with immune cell-specific ablation of different glucose transporters. We found that GLUT3-dependent glucose uptake controls a metabolic-epigenetic circuit that regulates the effector function of activated Th17 cells, in particular the expression of cytokines and chemokines. Metabolomic, epigenetic and transcriptomic analyses linked GLUT3 to mitochondrial glucose oxidation and acetyl-CoA generation as a rate-limiting step in the epigenetic regulation of inflammatory gene expression. How this metabolic pathway determines the progression and (long-term) immune responses to the commensal skin pathobiont S. aureus remains, however, elusive. The central goal of project C02 is to define the role of different glucose transporters in the innate and adaptive immune system and to investigate how intermediary glucose metabolism shapes immunity to S. aureus and prevents its spreading to secondary infection sites. In the following three aims, we will define and characterise the metabolic decision points in neutrophils and T cells, as paradigms of innate and adaptive immune cells, to contain and prevent cutaneous S. aureus infections.
Aim 1: Glycolytic regulation of T cell-mediated immunity to S. aureus infection.
Aim 2: Metabolic control of neutrophil responses to cutaneous S. aureus infections.
Aim 3: Defining the cellular and metabolic determinants of local S. aureus infection.








