rapidSTORM: accurate, fast open-source software for localization microscopy
04.12.2012Steve Wolter, Anna Löschberger, Thorge Holm, Sarah Aufmkolk, Marie-Christine Dabauvalle, Sebastian van de Linde & Markus Sauer
Nature Methods 9, 1040–1041 (2012)
Published online 06 November 2012 Corrected online 04 December 2012
In recent years, there has been a dramatic increase in the use of localization microscopy: that is, super-resolution fluorescence imaging by stochastic photoswitching, photoactivation or other fluorescence-generating processes. The evaluation of localization microscopy data with a Gaussian model of the point-spread function (PSF) is a common and cogent technique1, and Poissonian maximum-likelihood estimator (MLE) fitting of the Gaussian model has been shown to yield optimal precision2. However, current software support is based on less precise approximations of MLE fitting or is slow, hardware dependent, closed source or unmaintained. Many advances have been made with unpublished or prototypical software packages, leaving established labs with homemade software and new labs with immense technology entry costs. This state has weakened the core strengths of localization microscopy: cost-efficiency and experimental simplicity.
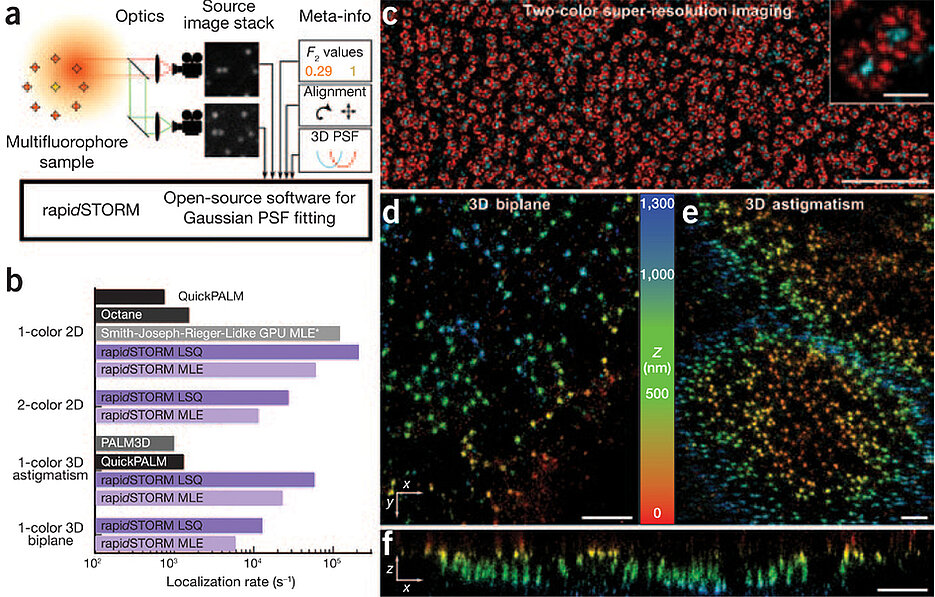
Here we report the computational optimization of iterative MLE fitting and the development of a fast, easy-to-use and open-source software program called 'rapid, accurate program implementing direct stochastic optical reconstruction microscopy'3 (rapidSTORM, Supplementary Software). To our knowledge, rapidSTORM is the fastest and most powerful open-source localization microscopy software available (Supplementary Table 1 and Fig. 1) and supports classic localization microscopy, astigmatic and multiplane three-dimensional (3D) assignment, and multicolor localization microscopy (Fig. 1). It is the only public software capable of quickly evaluating multiplane 3D and multicolor experiments. All variants are unified in a single fitting routine by integrating appropriate factors into the Gaussian PSF model (Supplementary Note 1 and Supplementary Fig. 1), thereby avoiding the introduction of unnecessary degrees of fitting freedom and allowing fitting of the emitter position in all planes concurrently with optimal precision4. We demonstrate the accuracy, ease of use and power of our approach with a range of 3D and two-color dSTORM experiments on the nuclear pore complex5 (Fig. 1c–f and Supplementary Fig. 2).
(a) The signal of single fluorophores is imaged on two spectrally separated cameras. Fluorophores are localized with a multispectral Gaussian PSF model and distinguished by their F2 values (the ratio of the intensity in the long-wavelength channel and the total intensity). (b) Speed of data processing with rapidSTORM and major alternatives. The bar groups denote different evaluation requirements, and the bar length shows the logarithm of the achieved speed. An asterisk denotes that displayed speed excludes preprocessing time; the software is not public. (c) dSTORM image of the nuclear pore complexes of Xenopus laevis oocytes5 shows the Alexa 700–labeled central channel (cyan) and the Alexa 647–labeled protein gp210 (red). (d,e) Biplane (d) and astigmatic (e) 3D dSTORM images of nuclear pores of X. laevis A6 cells labeled with Mab414 and Alexa 647–labeled antibodies. (f) Axial section of 2 μm thickness from d. Scale bars, 2 μm (c), 200 nm (inset) and 1 μm (d–f).