Closed Projects
- GRK 640: Sensory photoreceptors in natural and artificial systems
- SFB 554: Mechanismen und Evolution des Arthropodenverhaltens: Gehirn - Individuum - Soziale Gruppe
- SFB 581: Molekulare Modelle für Erkrankungen des Nervensystems"-Molecular models of diseases in the nervous system
- Euclock: Entrainment of the Circadian Clock
- SFB 581: TP B 28 Störung im Schlaf-Wachverhalten verursacht durch Transmissions-defekte an dopaminergen udn serotonergen Tripartite Synapsen am Modell Drosophila
- SFB 1047 Insect timing: mechanisms, plasticity and interactions
- FP7-People-2012-ITN: INsecTIME
Chronobiology is the study of biological rhythms. The best understood cycles are those with circadian 24 hour periods that modulate the temporal dynamics of physiology and behaviour of all higher organisms and some bacteria. Four model organisms have been widely used to study the underlying biology of circadian clocks, Cyanobacteria, the fungus Neurospora, the fruitfly Drosophila, and the mouse. CINCHRON is an integrated European centre of excellence for the research training of researchers in the emerging multidisciplinary field of Comparative INsect CHRONobiology. The 2017 Nobel Prize for Medicine or Physiology was awarded jointly to three of our colleagues, Jeffrey Hall and Michael Rosbash (both at Brandeis University, Boston, USA) and Michael Young (Rockefeller University, New York, USA) for their work on the molecular dissection of the circadian clock in Drosophila. Our warmest congratulations to these outstanding fly molecular geneticists for their achievements, which is particularly heartfelt because seven of the principle investigators of CINCHRON have worked and published with Hall and Rosbash. Insect clocks, both circadian and seasonal, are vital for adaptation to the environment and recent global patterns of climate change mean that insect pests and vectors of disease are expanding their ranges into Europe. Consequently there is an urgent need to study the circadian clocks of these insects and how they synchronise to the environment. CINCHRON will contribute to the integration and cohesion of future European research efforts in solving pure and applied biological problems.
Host Institution: Wuerzburg University
Supervisor: Prof Charlotte Helfrich-Foerster
Project 1: Seasonal clock in D. littoralis
Objectives: To discover the role played by the circadian clock in night-length measurement and induction of diapause in the northern European fruifly D. littoralis. The role and distribution of clock proteins and relevant clock-related neuropeptides will be studied under different photoperiodic and temperature conditions and mutagenesis (CRISPR/Cas9) of canonical clock genes will be used to examine whether clock genes measure night-length (which mediates diapause).
Project 2: Seasonal clock in pea aphids
Objectives: To discover the role played by the circadian clock in the induction of diapause in the pea aphid Acyrthosiphon pisum. Circadian behaviour will be characterised as will neuronal clock gene expression both temporally and spatially. CRISPR/Cas9 mutagenesis will be used to investigate whether clock mutations disrupt diapause.
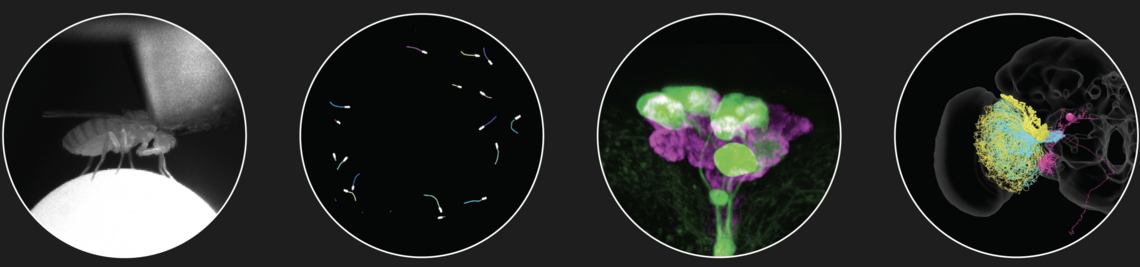
The ability to synchronize to the cyclical changes in the environment is a fundamental property of circadian clocks. To do so, they use the light-dark cycles as most important Zeitgeber and temperature cycles as the second Zeitgeber. However it is largely unknown how the two informations are integrated by the clock. The clock of the fruit fly consists of different interacting clock neurons that give rise to two activity peaks per day - one in the morning and the other in the evening. Light accelerates the oscillations of one group of the clock neurons whereas it slows down that of the others, so that the morning activity is advanced and the evening activity delayed under long summer days. This response is mediated by rhodopsins in the compound eyes. In addition the compound eyes mediate direct light responses of the flies, such as an immediate activity increase when lights are shut on. In this project we want to clarify the roles of the 6 different rhodopsins in the eyes (Rh1, Rh3, Rh4, Rh5, Rh6 und Rh7) for the different responses. Furthermore, we investigate how temperature cycles and light-dark cycles are integrated by the clock neurons.
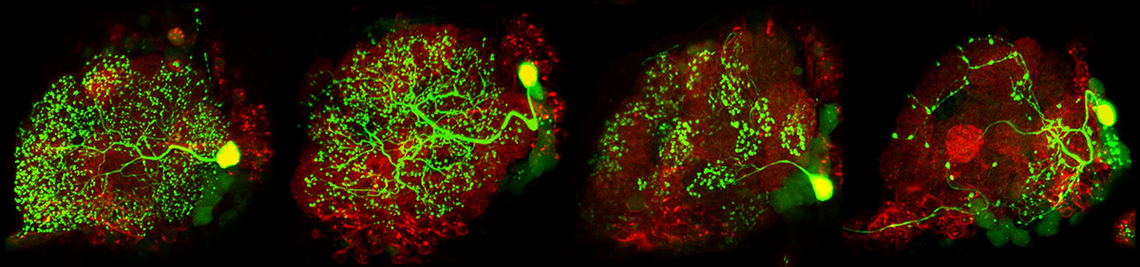
The fruit fly Drosophila melanogaster – is successfully used as model system to understand the function of endogenous clocks. As in mammals, several clock neurons interact in a neuronal network with neuropeptides as main communication signals. The best investigated neuropeptide in Drosophila’s clock is the "pigment-dispersing-factor" (PDF). PDF is expressed in 4 small and 4 large neurons per brain hemisphere and serves as coupling signal between the different clock neurons as well as as output signal to downstream neurons. Besides the PDF-positive neurons, neurons that express the short or long form of Neuropeptid F (NPF) and neurons expressing the Ion Transport Peptide (ITP) are important for the function of endogenous clock. The aim of the project is to classify their role in the circadian system and to unravel their action on other neurons by in vivo Ca2+ and cAMP-Imaging (Details see project of Christiane Hermann).
Chronic psychosocial stress and dys-regulation of the circadian clock share many common features. Both have dramatic consequences on health and life span, both prominently affect the HPA axis influencing glucocorticoid (GC) levels and immune responses, and both employ similar neuropeptides as signalling molecules. Anatomically, the HPA axis and the circadian clock in the suprachiasmatic nuclei (SCN) share a common interface - the paraventricular nucleus (PVN). Within the PVN, corticotrophin releasing factor (CRF) synthesizing neurons are stress responsive and trigger adrenal GC secretion. The PVN also receives inputs from the SCN, which regulates the circadian rhythm of GC secretion independent of stress. The daily GC peak prepares the animal for its active phase. This project will investigate the mutual interactions between psychosocial stress and the circadian clock in the SCN. We will determine (i) whether the circadian clock modulates the capability to cope with stress in a daytime dependent manner and (ii) whether psychosocial stress affects the circadian clock in dependence on the time of stressor exposure. To do so, we will subject mice to repeated social defeat (SD) stress in the morning or evening and investigate the physiological stress responses as well as the consequences on rhythmic behaviour and molecular circadian oscillations in the SCN.
The homeostasis of transmitters is essential for normal brain function and glia cells play an essential role in it. Disturbances of this homeostasis result for example in an abnormal sleep-wake pattern. This project will investigate the regulation of monamine release (specially of dopamine and serotonin) by postulated tripartite synapses between monaminergic neurons, glia cells and circadian clock neurons of the fruit fly Dosophila melanogaster.
In fruit flies, a mutation in the alanyltransferase "Ebony" that is exclusively expressed in glia cells results in disturbed sleep-wake patterns. Morphological investigations (confocal and EM) will show whether the postulated tripartite synapses exist. Sleep-wake studies of mutants with disturbed monamine transport and processing are planned to unravel the processes at synapses that lead to normal sleep. Ca++-imaging and period-luciferase imaging on cultivated brains will be performed to study the response of circadian clock neurons and glia cells to monamine transmitters as well as drugs that influence monaminergic signalling.
Animals living at high latitudes have to cope with prominent seasonal changes in their environment. In summer, they are exposed to long days and short nights with pleasant temperatures that allow reproduction, whereas the short days and low temperatures in winter require special adaptations to survive such as frost resistance and reproduction arrest.
In contrast, animals living close to the equator experience very little seasonal changes allowing reproduction throughout the year.
The circadian clock in the brain is known to control daily activity-rest rhythms and to provide an internal time reference for measuring day length. We found that the daily activity-rest rhythms and the neurochemistry of the clock network in the brain differs significantly in fruit fly species living at high and low latitudes and that these differences are causally related.
In order to understand circadian clock evolution we will investigate the clock network, the daily activity patterns of further fruit fly species living at different latitudes.
To understand the role of the circadian clock in day length measurement, we will use strains of Drosophila melanogaster caught at different latitudes. Our investigations will contribute to the understanding of circadian clock evolution by investigating fruit fly species adapted for a life at different latitudes.
The family of Drosophilids include tropical, cosmopolitan and subarctic species. The aims of the present study are to identify the neuronal clock mechanisms that are crucial for the adaptation of daily activity to different latitudes (project 1) and the molecular clock mechanisms that allow measuring day length and preparing for the winter (project 2). In project 1, we identified the neuronal clock network in the brain of the originally tropical Drosophila melanogaster as the ancestral one and that there are different ways to adapt the circadian system to a life at high latitudes, which all have one thing in common: they make the circadian clock more plastic so that the daily activity can easily follow the changing photoperiods. One way to do so is to lose the neuropeptide PDF (Pigment-Dispersing Factor) in some clock neurons and the blue-light photopigment CRY (Cryptochrome) in others (e.g. in flies of the virilis group). Other ways are to eliminate the rhythmic secretion of PDF (e.g. in Chymomyza flies) or to reduce the light-sensitivity of CRY or its ability to interact with the clock protein TIM (Timeless) (e.g. in D. funebris). In the second funding period, we aim to finish the already started analysis on ~30 Drosophila species stemming from different latitudes in order to gain deeper insights how circadian clocks evolved and adapted to the environment. Furthermore, we will focus on the clock of D. funebris because this clock appears unusally well adapted to a life at temperate and arctic regions and promises new insights into the evolution of high-latitude clocks. In project 2, we found that the distance between the maxima of the clock proteins PER (Period) and TIM in the clock neurons of D. melanogaster codes for day length and modulates frost resistance: under short photoperiods the maxima of these cycling clock proteins are close together and the flies are more frost resistant as under long photoperiods, in which the PER and TIM maxima are far apart. Furthermore, we identified 79 candidate genes that change their expression in specific clock neurons under short and long photoperiods. Now, we plan to identify the most promising genes by comparison of their expression patterns in high-latitude Drosophila species and D. suzukii, then manipulate them in D. melanogaster to study the flies’ thermal tolerance. Furthermore, we aim to identify the specific clock neurons in the brain of D. melanogaster that are most important for seasonal adaptation of frost resistance. This will not only allow a better understanding of the precise role of D. melanogaster’s clock in seasonality but possibly also unravel how the clock modifications in high-latitude species evolved. We will also generate per and tim null mutants in D. suzukii using the CRISPR-Cas9 technique to reveal the role of the circadian clock for seasonality in a fly with strong morphological and behavioural changes between summer and winter.
FO 207/16-1
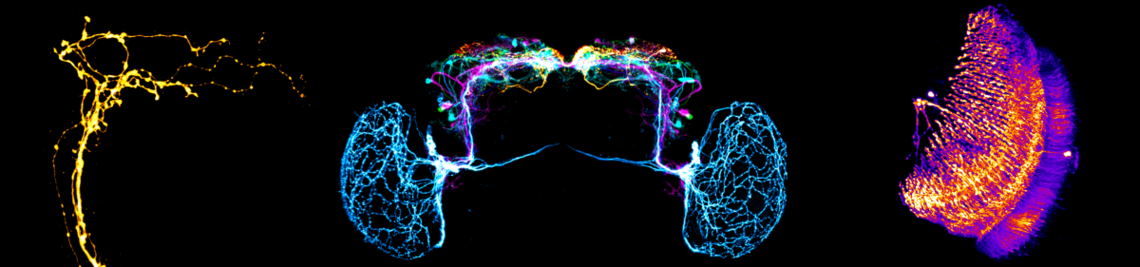
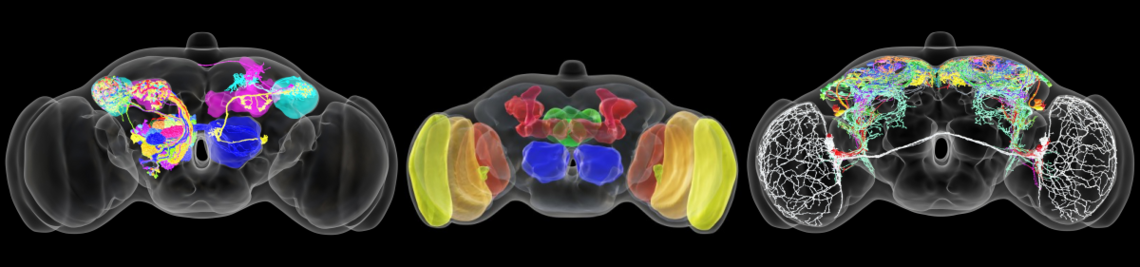
The circadian clock enables animals to be prepared in advance for the regular changes between day and night. Furthermore, some animals use their circadian clock for the memory of time, the measurement of day length (to anticipate seasonal changes) and for time-compensated sky compass orientation. The circadian clock network in the brain of the fruit fly belongs to the best-investigated. It consists of interconnected lateral and dorsal clock neurons, which generate circadian molecular oscillations and mediate these to downstream interneurons and neurosecretory centres in the dorsal protocerebrum. During the last years the fruit fly became a model in
sleep research; the first connections from the circadian clock to metabolism have been established, it was demonstrated that fruit flies have a time memory and that the clock is involved in diapause induction. Even the first hints exist that fruit flies are able to perform time-compensated sky compass orientation. The dorsal clock neurons appear to be involved in all these clock functions. While the lateral neurons represent “master oscillators”, the dorsal neurons are multimodal integrators that are essential for transferring the circadian rhythms to downstream interneurons and neurosecretory cells. Nevertheless, recent studies are controversial concerning the exact role of the dorsal neurons. Some authors claim that a special group of dorsal neurons (the DN1p) is responsible for sleep, whereas others postulate a role of the same neurons in arousal and elevated metabolism. The reason for these contradicting views lies most probably in the diverse neuronal projections of individual DN1p neurons. We could show that the dorsal neurons represent extremely heterogeneous groups of cells, but that their neuronal projections are still insufficiently characterized.
FO 207/17-1
Virtually all living beings on our planet exhibit daily and seasonal rhythms. These rhythms are generated by endogenous clocks, which allow organisms, including humans to synchronize daily and seasonal life cycle functions with rhythmic changes of their environment However, our current molecular understanding of biological rhythms and clocks is mostly restricted to land model species. By contrast, we know very little about the endogenous clocks of marine organisms, and how they interact with environmental cycles. This is particularly true for marine ecological keystone species like Antarctic krill (Euphausia superba), endemic to the Southern Ocean, a high latitude region which is characterized by extreme environmental changes across seasons (day length, light intensity, food availability). These polar regions are experiencing the fastest warming on the planet, with environmental alterations and ecosystem shifts, resulting in profound changes in trophic interactions and nutrient/energy fluxes. These finely tuned interactions, which have evolved over millions of years, are likely to going out of phase by the rapid climate change in these regions. Therefore, our overarching objective is to identify how regular environmental cues (day/night cycle, photoperiod) generate molecular rhythmic oscillations that allow polar marine organisms like Antarctic krill to anticipate the rhythmic changes in their environment, and to pre-adjust their life cycle functions accordingly. To achieve this, we aim to further investigate the involvement of endogenous clocks into central life cycle functions in Antarctic krill, by use of seasonal behavioural experiments along with gene expression analysis of clock and metabolic marker genes. In addition, we aim to characterize the location and anatomy of the master circadian clock in the brain of E. superba by use of fluorescent in-situ hybridization and immunocytochemical studies to understand the molecular and neuronal mechanisms that underly the endogenous clock. Finally, we will experimentally manipulate the circadian clock to reveal to which extent the endogenous rhythm and the changing environment determine the behavior and physiology of Antarctic krill. We thus hope to get further insights into the mechanisms that underly krill’s adaptation to extreme environmental conditions and into its plasticity towards ongoing changes in the Southern Ocean ecosystem.
More...
FO 207/19-1
The mitogen activated protein kinase (MAPK) ERK is a component of the eponymous signaling pathway that regulates various intracellular functions. Though typically associated with cell proliferation, differentiation and apoptosis, ERK also regulates many other processes, including neuronal and circadian mechanisms. Consequently, deregulation of ERK signaling does not only play a prominent role in tumorigenesis, but also in neuronal dysfunction and neuropsychiatric disorders.RSK proteins act as one out of several downstream mediators of ERK with apparently pleiotropic - but still poorly understood- functions in the nervous system. This is unfortunately illustrated by our lack of knowledge in pathophysiology leading to severe mental disabilities caused by rsk2 mutations in humans (Coffin-Lowry-Syndrome). This application aims to dissect neuronal RSK functions in Drosophila melanogaster, using the circadian system as a very well characterized experimental model. The clock network is ideally suited for an integrative approach because it allows analysis of RSK function at the molecular level, to study its impact on cellular/physiological processes and to evaluate its influence on behaviors. Based on our previous findings, we will first extend our analysis of RSK as a regulator of the molecular circadian oscillator. Second, promising first experiments support a function of RSK as a modulator of diurnal morphological plasticity of the dorsal terminals of a subclass of clock neurons (s-LNv). In combination with our previous findings in the motoneuron system of the fly, the question of RSK function in relation to synaptic properties and ERK signaling at these terminals arises. Does loss of RSK function alter s-LNv connectivity, and does this correlate with changes in time-dependent behavioral responses? A third focus of this application builds on our recent discovery of RSK function in fear-like behavior. We aim to dissect the RSK-dependent neural circuitry and the circadian modulation of fear-like behavior, and we will determine to which degree RSK-dependent modulation of G-protein coupled receptor signaling is involved. Given the similarities in neurochemical and molecular pathways between flies and humans, functions of RSK uncovered within the proposed fly project may not only provide a blueprint for RSK functions in mammals, but may also help to understand the complex pathophysiology of Coffin-Lowry-Syndrome.
Animals living at high latitudes have to cope with prominent seasonal changes in their environment. In summer, they are exposed to long days and short nights with pleasant temperatures that allow reproduction, whereas the short days and low temperatures in winter require special adaptations to survive such as frost resistance and reproduction arrest.
In contrast, animals living close to the equator experience very little seasonal changes allowing reproduction throughout the year. The circadian clock in the brain is known to control daily activity-rest rhythms and to provide an internal time reference for measuring day length. The latter is essential for a timely preparation for the winter.
We found that the molecular oscillations of the clock proteins Period (PER) and Timeless (TIM) in the brain of the fly differ under long summer and short winter days. Most interestingly, these flies also respond to short days with a change in the composition of their hydrocarbon (CHC) profile on the surface of the.
In order to understand the role of the circadian clock in day length measurements, we will use lab and wild-caught strains D. melanogaster. We will especially test whether there is a causal correlation between day length, PER/TIM oscillations, and cold resistance by investigating clock mutants that cannot normally adapt their clock to the seasons but remain in a quasi-permanent clock-winter- or clock-summer- state.
Our investigations will provide the first basis in understanding the role of the circadian clock in seasonal adaptation using the well characterized model D. melanogaster.
The circadian clock enables animals to be prepared in advance for the regular changes between day and night. Furthermore, some animals use their circadian clock for the memory of time, the measurement of day length (to anticipate seasonal changes) and for time-compensated sky compass orientation. The circadian clock network in the brain of the fruit fly belongs to the best-investigated. It consists of interconnected lateral and dorsal clock neurons, which generate circadian molecular oscillations and mediate these to downstream interneurons and neurosecretory centres in the dorsal protocerebrum. During the last years the fruit fly became a model in
sleep research; the first connections from the circadian clock to metabolism have been established, it was demonstrated that fruit flies have a time memory and that the clock is involved in diapause induction. Even the first hints exist that fruit flies are able to perform time-compensated sky compass orientation. The dorsal clock neurons appear to be involved in all these clock functions. While the lateral neurons represent “master oscillators”, the dorsal neurons are multimodal integrators that are essential for transferring the circadian rhythms to downstream interneurons and neurosecretory cells. Nevertheless, recent studies are controversial concerning the exact role of the dorsal neurons. Some authors claim that a special group of dorsal neurons (the DN1p) is responsible for sleep, whereas others postulate a role of the same neurons in arousal and elevated metabolism. The reason for these contradicting views lies most probably in the diverse neuronal projections of individual DN1p neurons. We could show that the dorsal neurons represent extremely heterogeneous groups of cells, but that their neuronal projections are still insufficiently characterized.
For animals, it is vitally important to time and synchronise development and physiological activity of the different body systems and to adjust behaviour accordingly. The timing and synchronisation of different body systems and behaviour requires both a timer (represented by central and peripheral endogenous clocks) and an integrating communication system (represented by the (neuro)endocrine system). Yet, we know astonishingly little about the complex neuronal and endocrine pathways and underlying molecular and cellular signalling mechanisms by which endogenous clocks and neuroendocrine systems interact with each other. Such knowledge could provide a handle to understand and treat associated developmental disorders and circadian dysfunctions including impaired fertility, sleep disturbances and psychiatric problems that can result from long-term disruption of this integrated timing. The aim of this project is to dissect the interactions between developmental and circadian timers and the neuroendocrine system, and to start characterising cellular and molecular signalling principles underlying timed behaviour. A main focus here is on the interplay between peptide and steroid hormone signalling and its circadian control. We also aim to find out where in the brain circadian and developmental signals are integrated to time a specific behaviour. This specific behaviour will be the eclosion of the fruit fly Drosophila. The fruit fly appears a highly suited model system for this project as it is genetically and experimentally well tractable, and possesses a relatively low number of individually identifiable neurons. This in the long range offers the possibility to in completeness decipher neuronal and hormonal connections between central and peripheral clocks and target tissues from the molecular to the systemic level. Eclosion is well suited as it is timed by developmental and circadian timers, and its timing is under control of both peptide and steroid hormones. From a zoological point of view, understanding the architecture of the neuronal-endocrine network timing eclosion is of great interest since correct eclosion timing is most critical for the survival and fitness of insects and other arthropods, which represent the vast majority of animals on our planet.
The mitogen activated protein kinase (MAPK) ERK is a component of the eponymous signaling pathway that regulates various intracellular functions. Though typically associated with cell proliferation, differentiation and apoptosis, ERK also regulates many other processes, including neuronal and circadian mechanisms. Consequently, deregulation of ERK signaling does not only play a prominent role in tumorigenesis, but also in neuronal dysfunction and neuropsychiatric disorders.RSK proteins act as one out of several downstream mediators of ERK with apparently pleiotropic - but still poorly understood- functions in the nervous system. This is unfortunately illustrated by our lack of knowledge in pathophysiology leading to severe mental disabilities caused by rsk2 mutations in humans (Coffin-Lowry-Syndrome). This application aims to dissect neuronal RSK functions in Drosophila melanogaster, using the circadian system as a very well characterized experimental model. The clock network is ideally suited for an integrative approach because it allows analysis of RSK function at the molecular level, to study its impact on cellular/physiological processes and to evaluate its influence on behaviors. Based on our previous findings, we will first extend our analysis of RSK as a regulator of the molecular circadian oscillator. Second, promising first experiments support a function of RSK as a modulator of diurnal morphological plasticity of the dorsal terminals of a subclass of clock neurons (s-LNv). In combination with our previous findings in the motoneuron system of the fly, the question of RSK function in relation to synaptic properties and ERK signaling at these terminals arises. Does loss of RSK function alter s-LNv connectivity, and does this correlate with changes in time-dependent behavioral responses? A third focus of this application builds on our recent discovery of RSK function in fear-like behavior. We aim to dissect the RSK-dependent neural circuitry and the circadian modulation of fear-like behavior, and we will determine to which degree RSK-dependent modulation of G-protein coupled receptor signaling is involved. Given the similarities in neurochemical and molecular pathways between flies and humans, functions of RSK uncovered within the proposed fly project may not only provide a blueprint for RSK functions in mammals, but may also help to understand the complex pathophysiology of Coffin-Lowry-Syndrome.