Signaling Networks in Cross-Striated Muscle Cells
Cross-striated muscle cells constantly withstand mechanical forces. During acute mechanical stress, protein signaling networks and proteostasis pathways are instantly activated and precisely regulated to meet and maintain the mechanical demands of contracting muscle cells.
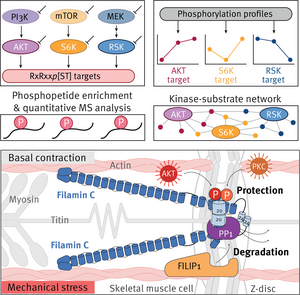
In this research focus, we systematically delineate phosphorylation-dependent signaling pathways and identify their specific targets in mechanically stressed myocytes. Phospho-signaling networks entail reversible and site-specific phosphorylation reactions controlled by kinases and protein phosphatases to determine cell functions and fate. To delineate such large protein phosphorylation networks, we use global and targeted quantitative phosphoproteomics approaches combined with high-end mass spectrometry technology. Based on our myotube phosphoproteome data, we have identified the myofibrillar Z-disc as a protein phosphorylation hotspot in cross-striated muscle cells. Specifically, we are investigating the complex regulation of the myocyte signaling adaptor and mechanosensitive protein filamin C (FLNC). This large actin-crosslinking protein is essential for the formation, the integrity, and the maintenance of sarcomeres. Notably, FLNC gene mutations cause severe myopathies and cardiomyopathies in humans, thus highlighting the high relevance of our research for human health and disease.
In current work, we want to gain a mechanistic understanding of FLNC’s function and regulation both under basal and acute mechanical stress conditions. So far, we have identified >30 mechanical stress-regulated FLNC phosphosites. Furthermore, we discovered that FLNC’s numerous protein interactions as well as its dynamics and turnover are tightly regulated by different protein kinases and protein phosphatases through site-specific phosphorylation/dephosphorylation reactions. For example, we have revealed that the FLNC-FILIP1 interaction is phospho-regulated: While FLNC is shielded from FILIP1 binding through phosphorylations by AKT and PKCa, it is rapidly dephosphorylated by PP1 under acute mechanical stress to promote FILIP1-mediated FLNC degradation. We propose that precise phospho-regulation of FLNC is key to stabilize and maintain sarcomeres in skeletal muscle cells during acute mechanical stress, which we are currently exploring in detail.
This research line of the Warscheid lab has been funded in the framework of the DFG Collaborative Research Units
-
FOR 2743 “Mechanical Stress Protection”, project 09 “Regulation of kinase-substrate networks in skeletal muscle cells under mechanical stress” https://www.biologie.uni-bonn.de/for2743/de
-
FOR 1352 ‘Structure, Function and Regulation of the Myofibrillar Z-disc Interactome’ project 04: ‘Study of Z-disc protein interaction and signaling networks’ (completed)
Selected Publications
-
 Filamin C dimerisation is regulated by HSPB7. . In Nature Communications, 16(1), p. 4090. 2025.
Filamin C dimerisation is regulated by HSPB7. . In Nature Communications, 16(1), p. 4090. 2025.- [ DOI ]
-
 Protein phosphatase-1 regulates the binding of filamin C to FILIP1 in cultured skeletal muscle cells under mechanical stress. . In Scientific Reports, 14(1), p. 27348. 2024.
Protein phosphatase-1 regulates the binding of filamin C to FILIP1 in cultured skeletal muscle cells under mechanical stress. . In Scientific Reports, 14(1), p. 27348. 2024.- [ DOI ]
-
 Identification of phosphatases that dephosphorylate the co-chaperone BAG3. . In Life Science Alliance, 8(2), p. e202402734. Life Science Alliance, LLC, 2024.
Identification of phosphatases that dephosphorylate the co-chaperone BAG3. . In Life Science Alliance, 8(2), p. e202402734. Life Science Alliance, LLC, 2024.- [ DOI ]
-
 Cardiac stress leads to regulation of Filamin C dimerisation via an ancient phosphorylation-modulated interaction with HSPB7. . In bioRxiv. Cold Spring Harbor Laboratory, 2024.
Cardiac stress leads to regulation of Filamin C dimerisation via an ancient phosphorylation-modulated interaction with HSPB7. . In bioRxiv. Cold Spring Harbor Laboratory, 2024.- [ DOI ]
-
 Phosphoproteomics Profiling Defines a Target Landscape of the Basophilic Protein Kinases AKT, S6K, and RSK in Skeletal Myotubes. . In Journal of proteome research, 22(3), pp. 768–789. United States, 2023.
Phosphoproteomics Profiling Defines a Target Landscape of the Basophilic Protein Kinases AKT, S6K, and RSK in Skeletal Myotubes. . In Journal of proteome research, 22(3), pp. 768–789. United States, 2023.- [ DOI ]
-
 Maintaining Proteostasis under Mechanical Stress. . In EMBO reports, 22(8), p. e52507. England, 2021.
Maintaining Proteostasis under Mechanical Stress. . In EMBO reports, 22(8), p. e52507. England, 2021.- [ DOI ]
-
 Order from Disorder in the Sarcomere: FATZ Forms a Fuzzy but Tight Complex and Phase-Separated Condensates with \($\alpha$\)-Actinin. . In Science advances, 7(22). United States, 2021.
Order from Disorder in the Sarcomere: FATZ Forms a Fuzzy but Tight Complex and Phase-Separated Condensates with \($\alpha$\)-Actinin. . In Science advances, 7(22). United States, 2021.- [ DOI ]
-
 Phosphoproteomics Identifies Dual-Site Phosphorylation in an Extended Basophilic Motif Regulating FILIP1-mediated Degradation of Filamin-C. . In Communications biology, 3(1), p. 253. England, 2020.
Phosphoproteomics Identifies Dual-Site Phosphorylation in an Extended Basophilic Motif Regulating FILIP1-mediated Degradation of Filamin-C. . In Communications biology, 3(1), p. 253. England, 2020.- [ DOI ]
-
 Myofibrillar Z-discs Are a Protein Phosphorylation Hot Spot with Protein Kinase C (PKC α) Modulating Protein Dynamics. . In Molecular & cellular proteomics : MCP, 16(3), pp. 346–367. United States, 2017.
Myofibrillar Z-discs Are a Protein Phosphorylation Hot Spot with Protein Kinase C (PKC α) Modulating Protein Dynamics. . In Molecular & cellular proteomics : MCP, 16(3), pp. 346–367. United States, 2017.- [ DOI ]












